Clinical-quality data from everyday life
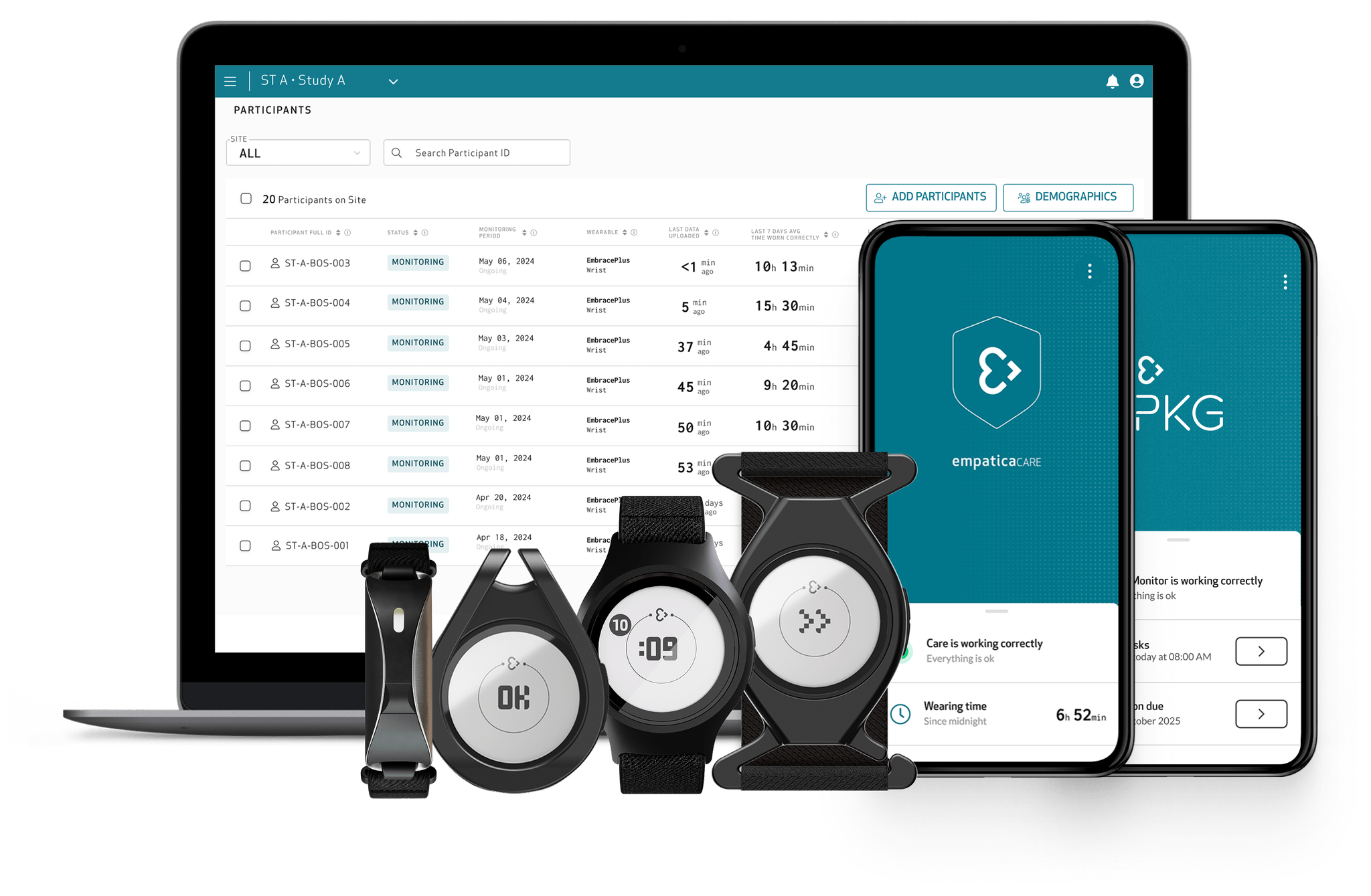
Introducing a new era in Parkinson’s monitoring
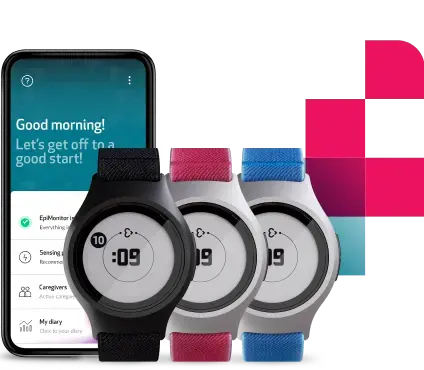
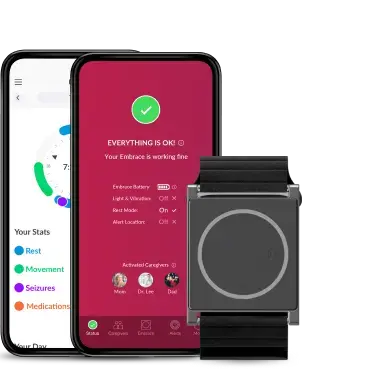
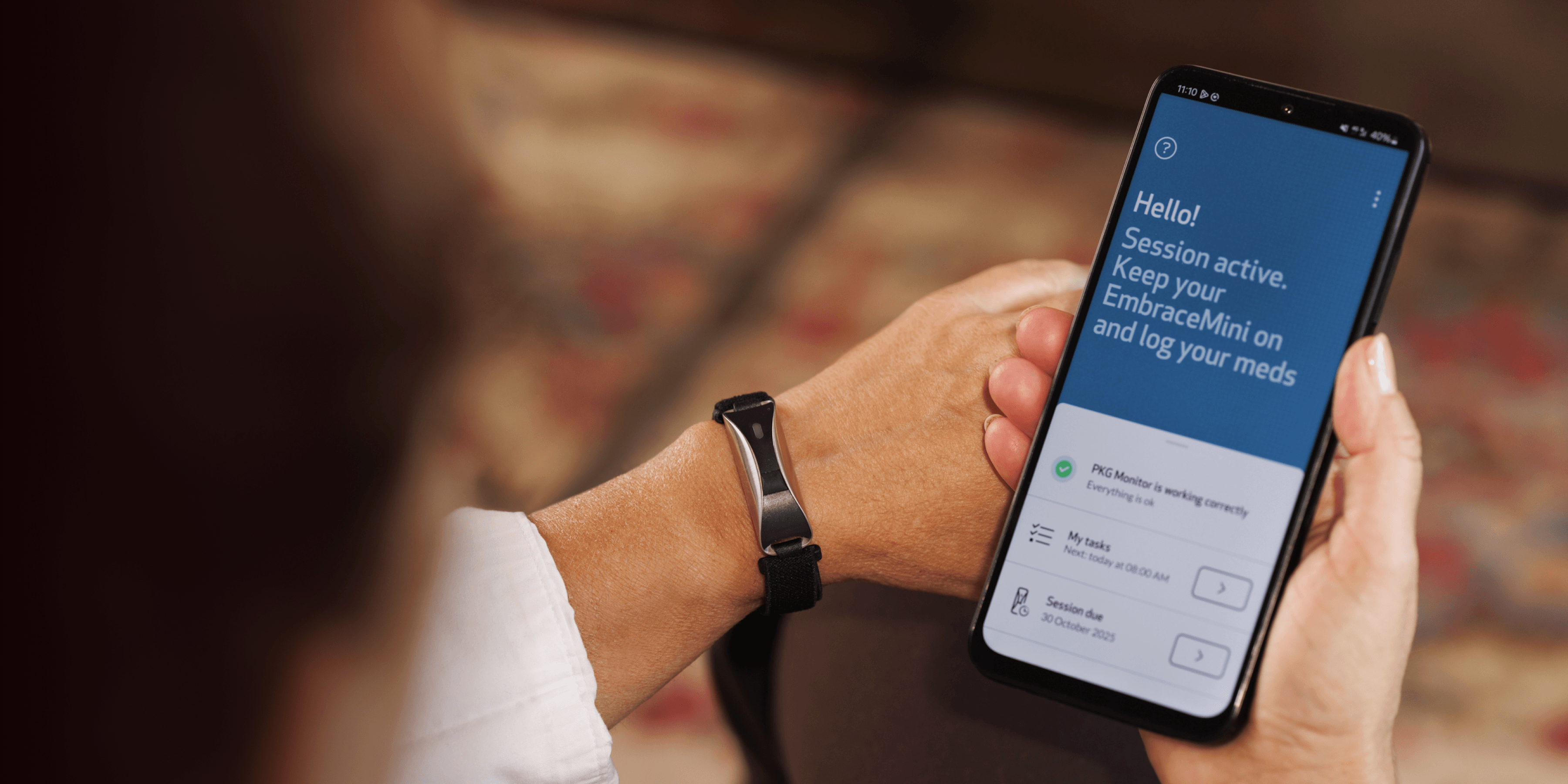
Empatica’s Parkinson’s Monitoring combines PKG’s trusted, clinically validated motor assessments with Empatica’s FDA-cleared and CE MDR certified wearable technology. The result: a modern, patient-friendly way to continuously track motor symptoms and therapy response, supporting more informed and personalized treatment decisions.
Learn more
7
FDA clearances
We have a track-record in producing the highest quality of medical devices
160+
countries available
Our products are currently available in over 40 languages and can be shipped globally. Local regulations may apply
100,000+
devices deployed
From patients to trial participants, Empatica's products have touched thousands of lives
300+
digital measures
The largest range of digital measures in a single solution. Already being used in hundreds of studies
12,000+
research papers
Empatica's technology has been referenced or used to publish thousands of academic papers
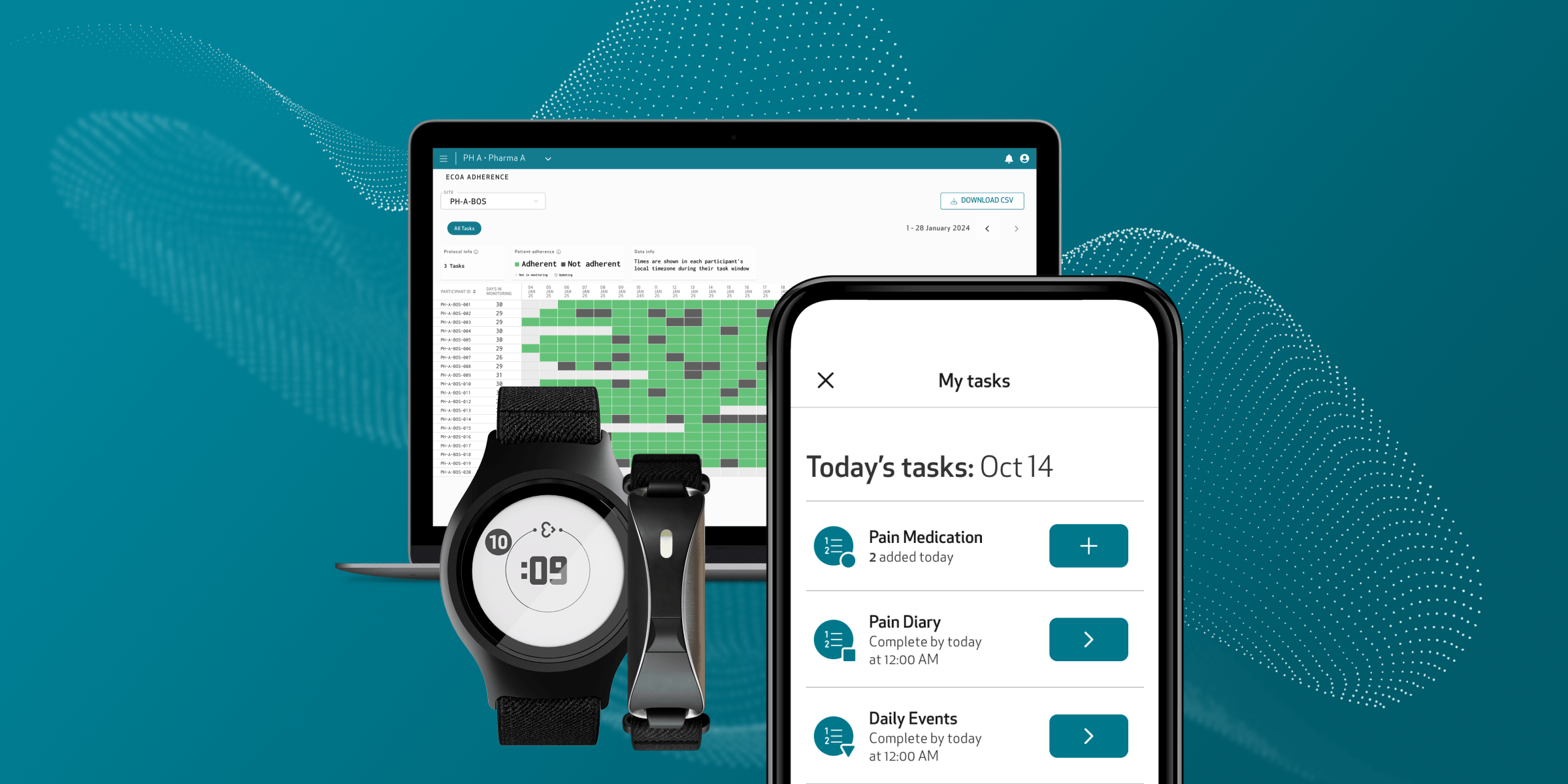
Redefining clinical research through medical wearables and digital biomarkers
More than half of the top 20 pharmaceutical companies use the Empatica Health Monitoring Platform to monitor the impact of treatments and to develop novel digital biomarkers that can be used as digital endpoints. Talk to our team to see how your research can benefit.

Get the latest from Empatica delivered directly
Still have questions?
Wondering how our platform can support your remote health monitoring and data collection needs?
Talk to our team