Latest FDA article endorses use of Digital Health Technologies in Clinical Trials
The latest FDA viewpoint confirms a growing shift across the Pharma industry: Digital Health Technologies (DHTs) are no longer a supplementary component—they’re becoming foundational to how trials are designed and evaluated.
DHTs enable data collection where patients live and work, reflecting real-life conditions and broadening access to clinical trials for more diverse and representative populations. They allow for continuous, passive monitoring of granular real-world endpoints—capturing everything from tremors to sleep to vital signs. This makes it possible to:
- Reduce participant burden;
- Improve patient retention by engaging participants throughout the clinical trial;
- Increase trial accessibility by enabling participation from individuals who may have been previously excluded, such as those with physical or cognitive impairments or living far from trial sites;
- Capture novel, meaningful outcomes to demonstrate improvements in how patients feel and function in their daily lives.
Importantly, the FDA recognizes digitally-derived endpoints as valid tools to support:
- Medical product registration;
- Label expansion;
- Long-term safety monitoring.
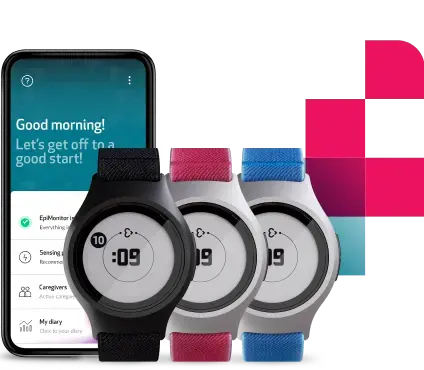
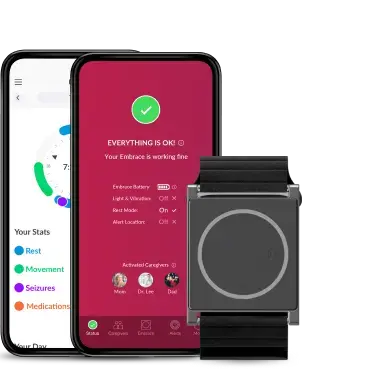
This approach is deeply aligned with how we have built our technology at Empatica. The Empatica Health Monitoring Platform and several of our digital biomarkers—including Pulse Rate, SpO2, Sleep, and Respiratory Rate—are already FDA 510(k) cleared, enabling the collection of validated, high-quality physiological data in real-world conditions. Our EmbracePlus wearable device, together with the digital biomarkers computed from its raw data, helps clinical trial sponsors:
- Run compliant, decentralized-ready clinical investigations;
- Measure validated, clinically meaningful physiological endpoints;
- Reduce participant burden while expanding access to trial participation.
The FDA encourages early engagement when integrating DHTs into trial design. We’re ready to support that journey, with technology built from the ground up for compliant, scalable deployment across clinical trials that includes remote monitoring, hybrid elements, or novel endpoints.
Learn how Empatica supports regulated clinical research here and visit our Digital Resources Library to access free white papers and guides on implementing digital endpoints and FDA-cleared technology.
If you want to know more, connect with our team of experts, who are here to support you through all the steps of adopting DHTs in your next trial.