How wearable technologies are enhancing clinical trials

Conducting site visits through telemedicine
Telemedicine has existed as a premise for decades, but the pandemic accelerated its adoption in a dizzying manner. Paired with a loosening of regulatory barriers, in order to enable remote access to healthcare while preserving the safety of patients and healthcare workers, the use of telehealth spiked. In the first weeks of COVID-19, and as the US declared a state of emergency, the number of Medicare members using telehealth grew 120 times. The first quarter of 2020 saw a 50% increase in the number of telehealth visits compared to the same period in 2019 (Source: CDC).
The embrace of the adoption of technology has been remarkable. A report by McKinsey mentions that providers are seeing 50 to 175 times the number of patients via telehealth than they did before. More importantly, from 11% in 2019, 76% of patients are now interested in using telehealth moving forward.
The success of clinical trials and drug development depends on the quality and quantity of data collected. Whereas in conventional trial setups the collection happens in the clinic, capturing only a snapshot of the patient’s health data (e.g. through an electrocardiogram), technology can help track the same data over large periods of time, capturing patterns that may be missed on-site, and paving the path towards new insights.
Technology is no longer a barrier, but an opportunity for running clinical trials. As patients are becoming more open to the modernization of interactions that have to do with their health, the adoption of technology to do so has multiplied. A world in which participants can attend their site visits without leaving the comfort of their homes, while site staff can interact with more patients and collect more data without adding to their workload, is already beginning to take shape.
Collecting data using digital devices
The adoption of digital devices allows researchers to collect data at home that they would usually only be able to collect in clinics. Wearable devices contain a range of sensors that enable the frequent, and even continuous collection of physiological signals from trial participants, while requiring minimal input from them. The data collected by wearable tech can be transmitted via an internet connection to sites or a centralized database, and from there it can be accessed by CROs or sponsors.
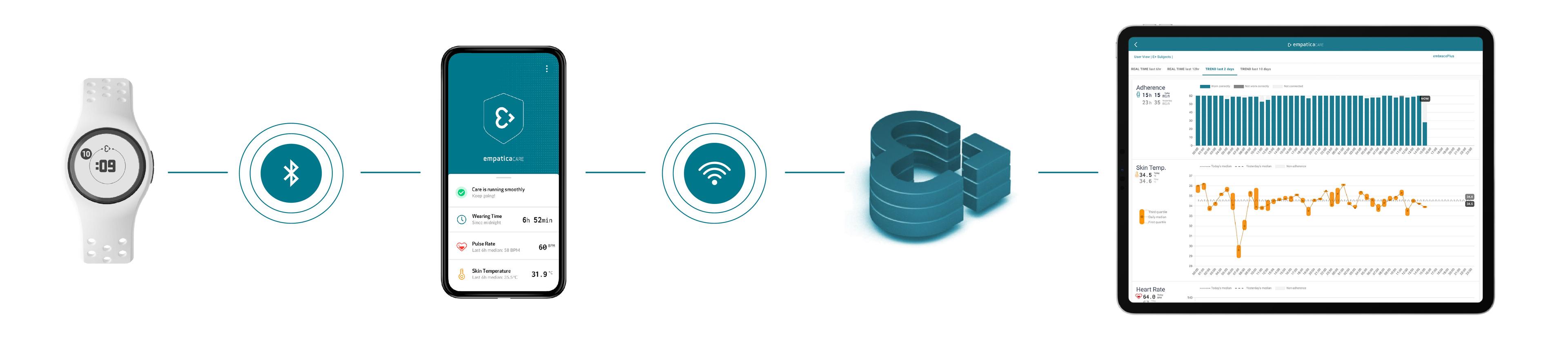
A device, in this example, our EmbracePlus, can collect data and send it to a smartphone app. The App can allow participants to check on the status of their wearable device while streaming data to the Cloud. Data can be visualized in online dashboards by site personnel, while it can also be downloaded in their raw format for further analysis.
Empatica’s wearable technology can seamlessly collect and stream data without participant or site personnel intervention.
Devices can improve participation and retention by overcoming site and patient burden, while also potentially providing more reliable data than what is often collected on-site. The most common version of wearable tech found in day-to-day life comes in the form of fitness trackers, but medical devices that resemble their consumer-friendly counterparts are beginning to emerge.
When evaluating wearable technologies for your trial, it is important to remember a few key factors that can help you make the best possible choice:
- Reliability Are there peer-reviewed papers and/or studies on clinicaltrials.gov that validate the specific technology? In addition, although this is not a requirement for use in clinical research, consider certifications by regulatory bodies like the FDA that can provide an additional layer of reliability.
- Quality Beyond price, consider the overall return on investment of the devices: the longevity of key components (for example, battery lifecycle), the build quality, any potential maintenance costs, the type of support offered throughout the study.
- Usability The overall usability of the device can be as important as its performance. Opt for devices that require removal as rarely as possible. Waterproof devices with extended battery lives, that are particularly comfortable to wear at night, can enhance data collection and offer truly round-the-clock data.
- Security As part of your research, look for HIPAA and/or GDPR-compliant solutions, that offer transparency on data ownership and storage.
- Accuracy How a device measures a data signal (e.g. the average of one minute, versus a snapshot taken once a minute), and the accuracy of the overall data against the endpoint it is used for, is a key factor for consideration.
- Scalability For truly multisite, borderless trials, consider solutions that can support multiple devices simultaneously, with cloud technology that can easily gather and store data remotely and wirelessly.
- Connectivity Diverse datasets largely depend on the openness of the technology adopted. 43% of US citizens have Android devices. Choose wearable devices and applications that can work across operating systems, but also device types (smartphones, tablets). The technology should be able to transfer data wirelessly, either via an internet connection or Bluetooth, to ensure easy, remote access for researchers.
- Data availability Data can come in many formats. Choose a provider that supplies the data based on the needs of your research. Consider the wearable tech that is used and the sensors and digital biomarkers provided, and how these match your research needs.