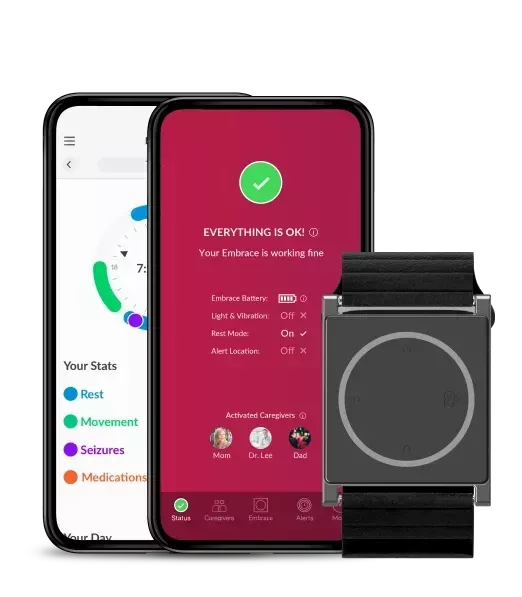
How to enhance compliance in clinical research with Embrace2

Subjects’ willingness to volunteer for studies on the effects of investigational agents, and diligence in following prescribed protocols are fundamental to clinical research.
However, once enrolled in clinical studies, some subjects incompletely execute their charge.
There are two primary types of adherence, or compliance, in research:
- Follow-up adherence refers to fulfilling the scheduled sequence of assessment measures within planned time windows (e.g. keeping follow-up clinic visits, undergoing laboratory tests) until reaching endpoint.
- Regimen adherence refers to pursuing the assigned regimen consistently (e.g. keeping to schedule in taking medication at the specified dose, maintenance of diet or exercise regimens, self-monitoring, meeting objective biological targets).
Adherence problems can be random or non-random (e.g., treatment effects, or length of time in study), transitory or long-term, or attributable to other factors with implications for data analysis and interpretation [1].
Research subjects’ intentional or inadvertent non-adherence, and deviations in protocol may threaten researchers’ ability to complete investigations.
Problematic adherence may lengthen studies, necessitate larger subject samples to achieve adequate statistical power, and inflate the costs of completing studies.
It has been estimated that it takes only 30% of participants to be less than fully adherent to require doubling the number of participants necessary to produce an equally significant study [2].
The adverse effects and costs of subject adherence for clinical research reveal a compelling need for more effective techniques for promoting, sustaining, and evaluating subject adherence through all research phases.
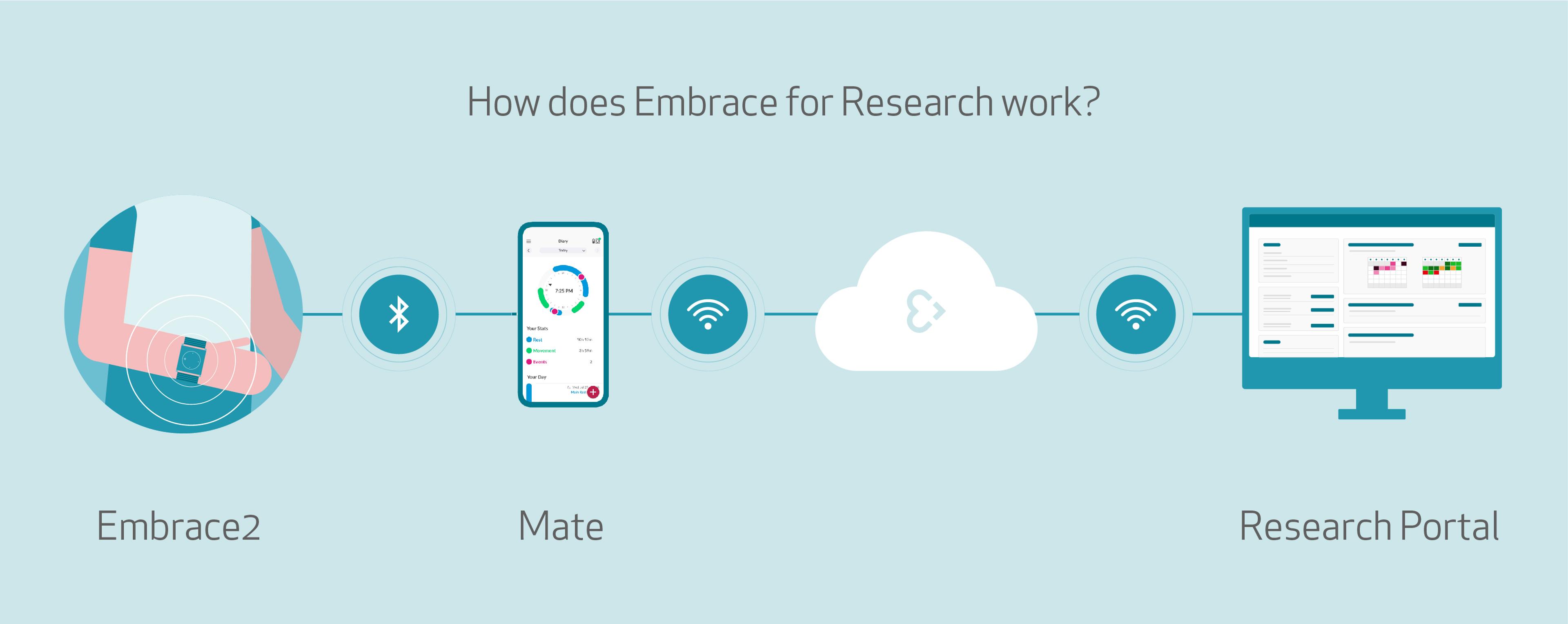
How does the Embrace2 for Research boost participants’ adherence?
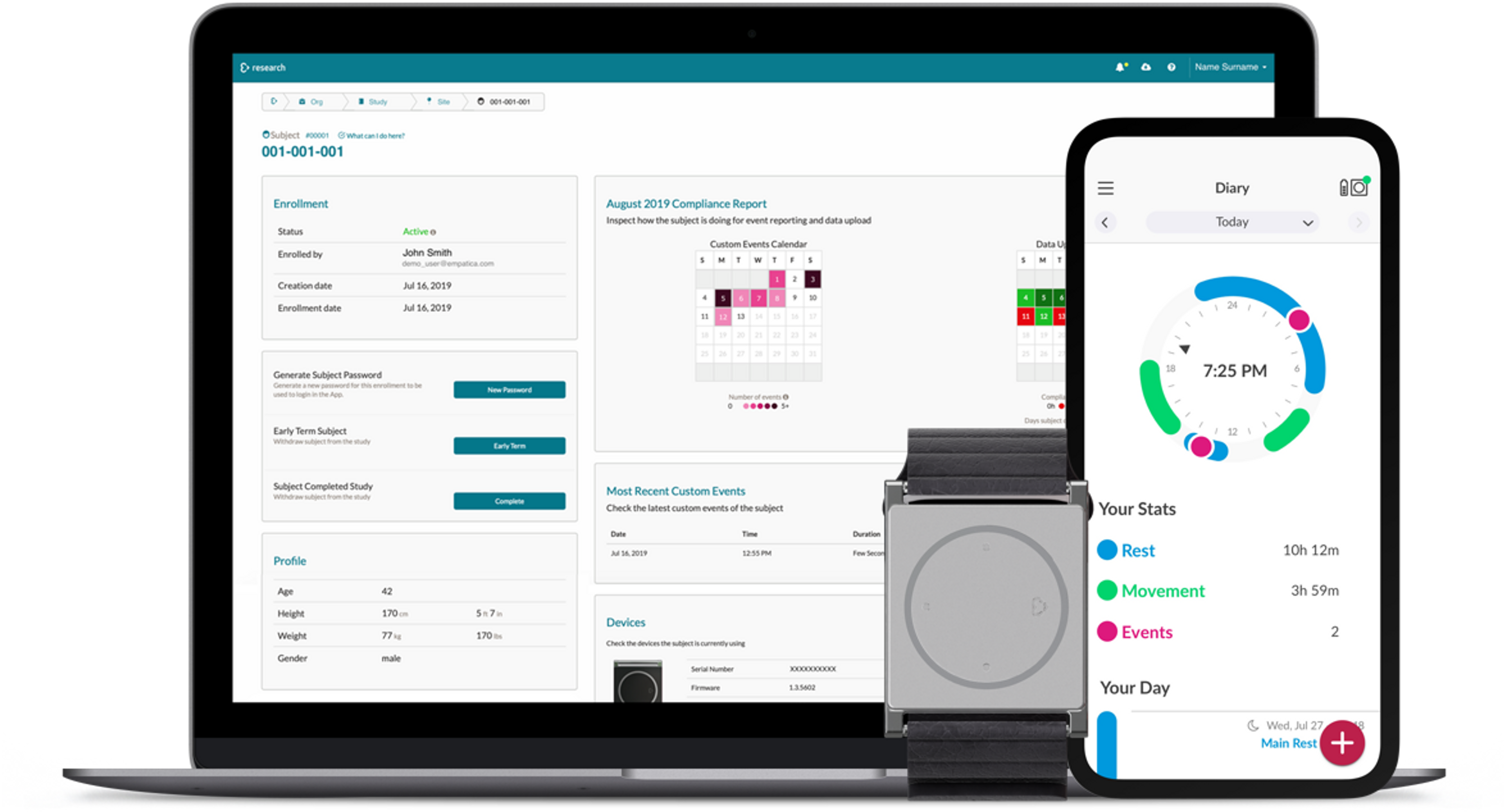
- It guarantees remote, automated real-world data gathering
Physical visits at hospitals or clinics are one of the most limiting factors in follow-up adherence.
The Embrace for Research guarantees clinically-validated data collection remotely, and automatically without the need for participants to check-in at the hospital.
How? The Embrace2 smart watch, worn by the study subjects, continuously collects and safely sends raw physiological and computed data through Bluetooth to a paired app: Mate.
Site coordinators, CROs, or researchers can access the continuously uploaded and protected sensor data through a cloud platform: the Research Portal.
- Its e-diary keeps participants engaged while delivering an objective data set
Noncompliance is often not intentional, and simply due to forgetfulness.
Paper-based diaries have traditionally been used to capture all the information needed from participants during a clinical trial such as the time when they took their medicine and how they felt at different times during the day. However, participants may forget to complete them on a daily basis [3] and, it takes time for the clinical trial staff to transfer all the information into an electronic form to be analyzed [4].
An electronic diary, like the one embedded in our MATE app, is able to accurately capture the time and date when an entry was made and so provide a more reliable source of information.
Subjects can manually add notes and mark relevant events, automatically delivered in a digital format for the trial staff.
A qualitatively optimized data set can lower variance and enhance study power thanks to clearer conclusions.
- It features a wearable that participants will want to wear
Embrace2’s sensors, which continuously measure physiological parameters, are packed in a consumer wearable device. Not only the Embrace has been appositely designed to be comfortably worn 24/7 by the study subjects, but it also features an extremely modern and stylish design that can make subjects less inclined to discontinue their adherence to the investigation.
- It’s incredibly easy to use
Another barrier to engagement is often the confusion over the protocol instructions or the use of new technology. It’s important that participants fully understand everything they have been asked to do.
We are proud to have designed an easy-to-use medical device, which also comes with an in-app onboarding process with instructions on its functionalities and comprehensive, quick start guides to ease the adoption of the technology and enhance subjects’ retention.
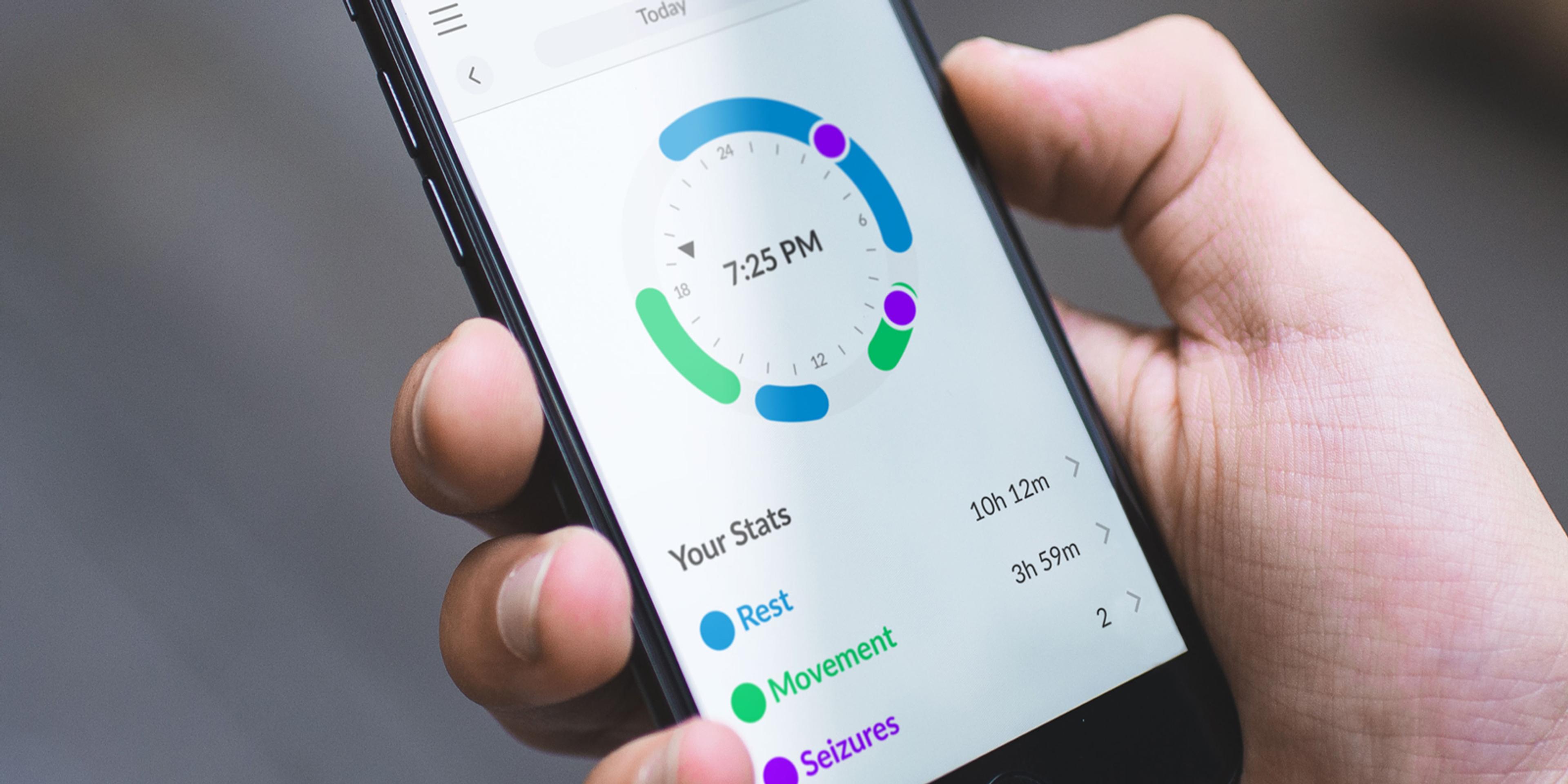
- Subjects can visualize their biodata thought the MATE app
MATE, functioning as an e-diary, also plays a role in augmenting subjects’ motivation and adherence.
The physiological data, gathered by the app, automatically uploaded to the Research Portal, is processed and sent back to the Mate App for visualization, so subjects can access an in-depth analysis of their rest and physical activity, encouraging subject engagement even more.
- It enables comprehensive monitoring of subjects’ compliance
Monitoring enables adherence-enhancing efforts to be targeted and allows for analysis of the effect of adherence on the outcomes of the research [5].
With the Research Portal, research teams, CRO personnel, and study coordinators have full control and a global overview of subject monitoring.
By spotting low-compliance issues in seconds, they can intervene early when adherence problems emerge, facilitating adherence itself, preventing lengthening the investigation, and mitigating costs.
Contending with non-adherence can be often demoralizing to staff and it can contribute to staff burnout and turnover. With a tool that makes the subject compliance more transparent and intelligible, study coordinators can be more informed. And, by understanding at a glance if and how often enrolled subjects are wearing the device correctly throughout the study, investigators can maximize the quality and quantity of data collected, ultimately promoting clinical outcomes, and facilitating scientific progress.

If you are interested in using Embrace2 and the Research Portal for your research study/clinical trial, you can get in touch with our team here.
Feel free to also visit our support page to learn more!
Words worth reading
Sources:
- https://edisciplinas.usp.br/pluginfile.php/2317858/mod_resource/content/1/
- https://www.ncbi.nlm.nih.gov/pubmed/3072187
- http://www.ncbi.nlm.nih.gov/pmc/articles/PMC111114/pdf/1193.pdf
- https://www.eupati.eu/clinical-development-and-trials/assessing-participant-adherence-during-clinical-trials/
- https://www.worldcat.org/title/compliance-in-health-care/oclc/4493661