Empatica Receives European MDR Certification for Health Monitoring Platform
Empatica is glad to announce it has received CE Mark Certification under the European Union’s Medical Device Regulation (MDR) for the Empatica Health Monitoring Platform. Providing over 300 digital measures, the Platform becomes the largest wearable and digital biomarker solution to achieve both CE MDR certification and FDA clearance, marking another significant step forward in providing clinical researchers with a unified, robust, and scalable solution for their global trials.
The Medical Device Regulation (MDR, EU 2017/745) sets a new benchmark for medical devices when compared to the older Medical Device Directive (MDD, 93/42/EEC), with more stringent requirements aimed at enhancing patient safety, transparency, and accountability within the EU market.
An important characteristic of the MDR certification is a more rigorous and robust process for product clinical evaluation, including more clinical evidence and data to demonstrate product safety and performance. In addition, a detailed and comprehensive approach to post-market surveillance ensures guaranteed functionality and accountability over time. This is especially crucial for organizations that are conducting clinical trials and seeking long-term, reliable, and future-proof solutions. Another key requirement of MDR is stricter conformity with all relevant safety and performance standards, meticulously documented to ensure compliance.
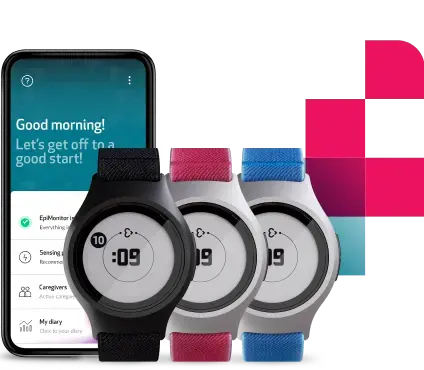
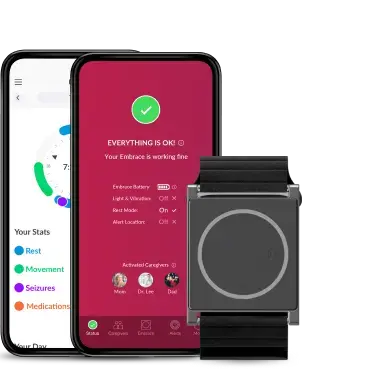
Already FDA-cleared since 2022, the Empatica Health Monitoring Platform provides researchers with the EmbracePlus wearable, a dedicated software suite, API integrations, and over 300 digital measures. EmbracePlus continuously captures real-world patient data using advanced digital biomarkers and streams these securely to the Cloud, supporting researchers in monitoring patient outcomes and evaluating the effectiveness of treatments in hybrid and decentralized trials.
With MDR certification, Empatica showcases its ongoing compliance with the most stringent international regulatory standards and reinforces its position as a trusted technology partner for pharmaceutical companies and contract research organizations (CROs).
Providing the most comprehensive platform for clinical trials that is both FDA-cleared and CE-marked under MDR is a milestone that further showcases Empatica’s continued commitment to providing truly global, medical-grade innovations for remote health monitoring and clinical research, with a focus on safety, security, and high-quality data.
Write to us and learn more about how the Empatica Health Monitoring Platform can redefine clinical care and research.
—
About Empatica
Empatica Inc is a pioneer in continuous, unobtrusive remote health monitoring driven by AI. Empatica's FDA-cleared platform and technology is deployed in over 160 countries and used by top pharmaceutical companies and research institutions, in studies examining stress, sleep, epilepsy, migraine, depression, addiction, and other conditions. Its flagship medical wearable, EmbracePlus, has been developed with key partners including HHS, USAMRDC, and the NASA-funded TRISH.