Empatica receives first of its kind European CE mark for early detection of COVID-19
Another milestone for wearable technology in the battle against COVID-19
After years of hard work, we are excited to announce that Empatica has received the CE mark for its respiratory infection detection system Aria, as well as its Empatica Care remote health monitoring platform.
CE marking is a seal of approval that shows a product has met the European Union’s safety, health, and environmental protection requirements, and can now be marketed and used across all EU Member States.
This makes Aria a world-first innovation, as there is no other commercially available medical product that automatically provides an early warning when the algorithm detects a possible respiratory infection.
Achieving the CE mark is the result of months of work from the Empatica team, which started in 2018 with our partnership with BARDA to develop wearable technology that could detect the onset of a respiratory infection. As the pandemic outbreak began, and with irrefutable evidence that COVID-19 was a disease that mainly affected the respiratory tract, particularly in more severe cases, we accelerated those efforts and sought to bring a product to market that could also help combat the spread and impact of the virus.
Automated and early detection of COVID-19 and other respiratory infections using Empatica's algorithm and wearables
Aria can achieve early detection of COVID-19 and influenza-like diseases with no input from its users. The system has been designed to be as easy to use as possible, for both the people wearing the devices and the professionals responsible for monitoring their health.
The system consists of:
- A respiratory infection detection algorithm, developed by Empatica
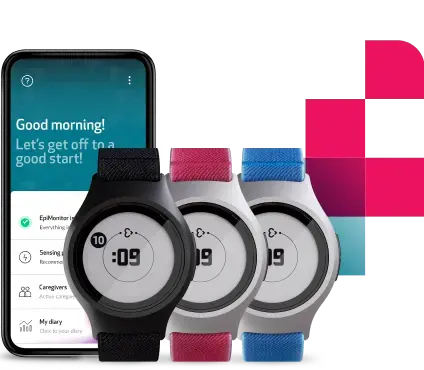
- An Empatica smartwatch equipped with powerful sensors that can monitor key vitals such as pulse rate, pulse rate variability, skin temperature, and electrodermal activity from the wrist (either the E4 or the EmbracePlus)
- A smartphone app (for the people whose health is being monitored)
- A cloud-based portal (for the professionals)
Aria provides a simple and user-friendly experience, without adding any additional burden to the individuals whose health is being monitored.
Data is collected seamlessly and passively by an E4 or EmbracePlus health smartwatch, which users can wear like any other watch while they go about their daily lives. Their data is continuously collected by sophisticated sensors, and it is analyzed by the algorithm and displayed inside the online portal and user app. All this process is done automatically and requires no input from the user.
How can the algorithm detect the presence of COVID-19 without visible symptoms?
The algorithm has been trained to spot any changes in the data of users that suggest their body is beginning to fight a respiratory infection. This is usually indicated in differences seen in pulse rate, pulse rate variability, but also electrodermal activity, the continuous measurement of which is a unique feature of our wearables. The Aria algorithm analyzes the data collected and displays a ‘risk warning’ when the observed patterns indicate that someone is developing an infection. If that is the case, they would be informed inside the app, while a professional would also be informed inside the portal, knowing whether they should intervene by suggesting a swab.
More importantly, the algorithm can detect the presence of infection even before any visible symptoms manifest, which can be crucial in limiting spread, especially considering that COVID-19 is at its most infectious earlier on in the illness.
For a more normal 'new normal'
COVID-19 has, to date, resulted in 2.5m deaths in Europe and 500k deaths in the US. As we begin to transition to the new normal, we are all counting on the ‘miracle’ of the vaccines to diminish the impact of the virus and enable some form of herd immunity to be reached. However, this remains a gargantuan task, and it is becoming widely accepted that vaccines will not be a silver bullet. The WHO has continuously iterated that vaccination will not end the pandemic, and we will all need to continue using every measure that is at our disposal to ensure future waves of this, and other, pandemics are prevented.
With the constant emergence of new variants for which there is no proof that existing vaccines can be effective, technology like Empatica Aria can provide a solution that offers an additional sense of security and safety as we transition towards the new normal, a normal in which the virus is not completely eradicated, but using the sophistication in science and technology that we have achieved as humanity, it can be kept at bay. For this, vaccines alone are not enough, particularly if vaccination becomes a seasonal requirement, similar to the flu.
Experts agree that the virus is likely to become endemic even though it could, hopefully, be less of a threat as time passes. Despite that, we are entering a transition period, and using innovation that can function alongside vaccines, testing and -hopefully- treatments, can ensure that we are prepared to face the uncertainty and rebuild our normality.
Thankfully, Empatica is poised to stand alongside the great leaps made in science and technology throughout the past year. With its ability to detect respiratory infections early, and the simultaneous communication of this output to both the user and a provider, Aria can allow early self-isolation and intervention, ensuring both the infected person and their communities can be kept safe while minimizing transmission. Instead of using PCR testing for screening, utilizing a highly scalable screening tool like Empatica Care and Aria can fill a significant gap in the current approach to COVID screening, with the ability to effectively funnel those with a high risk of infection to the appropriate diagnostic test.
How accurate is Empatica Aria in detecting a respiratory infection?
The clinical performance of the artificial intelligence/machine learning algorithm of the Empatica Aria system has a sensitivity of 0.94. This means that the algorithm will be accurate in detecting the presence of a respiratory infection in a patient, even without any visible symptoms, in 94% of the cases. The results apply to general physiological responses that arose with three kinds of viruses: H1N1, Rhinovirus, and SARS-Cov2.
In fact, in Europe, these numbers are on par with the guidelines issued by the European Center of Disease and Control Prevention on the selection of reliable rapid antigen tests, which suggest that only rapid antigen tests with a sensitivity of 80% or higher should be used.
More importantly, the detection happens on average after 2.64 days from the inoculation of the virus. When it comes to COVID-19, since the most infectious period is earlier on in the illness, early detection can prove key in controlling outbreaks.
Who can use Empatica Aria?
According to the CE certification, Aria can be safely used by anyone aged 14 and above and is suitable for healthy individuals who may be at risk of developing a respiratory infection. The Empatica Care platform is intended for use by professionals in healthcare facilities and company environments.
Physicians can use Aria with Care to monitor not only the vitals of their patients but also to get valuable input by the algorithm in case someone’s data suggests they may require more attention because of developing an infection.
In environments where infectious diseases can spread rapidly, like universities, sports clubs, or even offices, Aria can prevent potential outbreaks by indicating who should self-isolate and seek testing, while ensuring that a healthcare professional will be informed and, paired with the vital signs monitoring that Empatica Care offers, will know if they need to intervene and offer additional care.
Can I use Empatica Aria in the US?
In June we announced that we had received further support from HHS/BARDA, and a few months later we also received an award by the US Army to make the Aria system available in the US. Our algorithm is currently under validation in the US with select partners in order to gain FDA approval.
If you would like to participate in the US nationwide validation trial, run in collaboration with Snyder Lab at Stanford University, visit this link. We are looking for healthcare workers based anywhere in the US, with frequent patient contact, who have not previously had COVID-19 or received the vaccine.
What is Empatica Care?
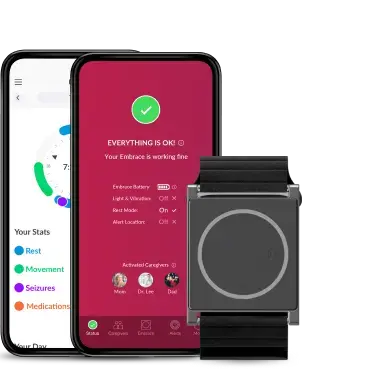
Empatica Care is our scalable remote health monitoring platform. It uses our wearable tech and cloud infrastructure to stream the vital signs and digital biomarkers of patients and healthy individuals in real-time, so it can be accessed by a professional.
The platform consists of an Empatica health smartwatch (the EmbracePlus) with advanced sensors for physiological data collection, a cloud-based portal for healthcare providers and professionals to access this data, and a smartphone app for the users, that visualizes and streams the data and biomarkers monitored to the portal. We’ve designed it so it can be a completely automated and passive experience for the users, who only have to wear a device and make sure they are running the Care App on their smartphone and remain connected to the internet.
You can take a closer look at Empatica Care in our dedicated blog post.